ADVERTISEMENT
An Appraisal of Potential Drug Interactions Regarding Hyperbaric Oxygen Therapy and Frequently Prescribed Medications
Index: WOUNDS 2011;23(6):147–159
Abstract: Many healthcare providers may overlook or even be unaware of most drug-to-drug interactions. Recognizing the existence of drug interactions with the use of hyperbaric oxygen can empower a clinician with knowledge to avoid dangerous interactions that may result in hazardous, negative patient outcomes. Hyperbaric oxygen therapy (HBOT) can reduce the efficiency of certain drugs or make drug therapy more unpredictable. Methods. This review offers the physician information regarding prescription drug interactions with hyperbaric oxygen therapy. First, mechanisms found in the medical literature of potential drug interactions with the use of hyperbaric oxygen are presented. Second, the 100 most frequently prescribed medications in 2009 are reviewed regarding hyperbaric oxygen. Lastly, a table of these 100 medications and any reported effects of hyperbaric oxygen on each drug are provided. Results. The total number of different medications in this review was 69. Reported drug interactions resulting from the effects of hyperbaric oxygen occurred with 38 of the 69 drugs that were reviewed (55%). Descriptions of the possible effects of hyperbaric oxygen are presented for each reviewed medication. Thirty-one medications of the 69 review drugs (44.9%) did not have any description of the possible effects of hyperbaric oxygen. A few references recommended avoidance of hyperbaric oxygen because co-administration of these drugs predisposes the patient to oxygen toxicity. Conclusion. Hyperbaric oxygen therapy may interact with medications through pharmacokinetic or pharmacodynamic mechanisms. This review offers the healthcare provider information regarding potential drug interactions. Empowered with this information, clinicians may assist their patients to maximize pharmacologic outcomes by avoiding these reported harmful interactions. Prescription medications are vital to preventing and treating illness as well as assisting in the avoidance of more costly medical complications.1 Use of prescription medications to treat chronic medical conditions is particularly high among older individuals. Almost 40% of older Americans take five or more therapeutic agents monthly.2 Moreover, the most recent data from a sample population survey of United States civilian households reveals that 50% of the population consumes at least one or more prescription drugs per month, while 1 out of 10 Americans use five or more prescription drugs each month.2 Oxygen is a common and widely prescribed therapeutic agent. Given, oxygen possess both biochemical and physiological actions, a distinct range of effective doses, and well-defined adverse effects at high doses, it may be considered a pharmacologic agent.3 Hyperbaric oxygen (HBO) therapy is defined as inhalation of oxygen at increased pressure, for potential therapeutic benefit in a variety of clinical situations.4 Medical literature and current compendia have reported the use of hyperbaric oxygen therapy to treat hypoxic as well as diabetic chronic lower extremity ulcers as an effective adjunctive wound treatment.5–10 Upon reflection of lower extremity ulcer prevalence statistics collectively, as well as prescription use, patterns with the inclusion of hyperbaric oxygen therapy encourage an inference in which overlapping cross-sectional population exists as it pertains to prescription medications and patients with lower extremity wounds being treated with hyperbaric oxygen under pressure. Many healthcare providers may overlook or are unaware of specific potential drug interactions. It is imperative that clinicians be knowledgeable of the existence of pharmacological interactions either beneficial or harmful between a patient’s medication regimen and potential outcome effects of hyperbaric oxygen effects.  The purpose of this review is to offer clinicians information regarding oxygen drug and physiology effects within the context of HBOT, increased atmospheric pressure, and lower extremity wound care. In order to accomplish this endeavor, specific concepts must be exemplified. An overview describing human pharmacokinetics, observed human physiological effects of hyperbaric oxygenation and pressure will be first offered because achieving this understanding is essential when discussing both pharmacodynamic and pharmacokinetic principles of drug-drug interactions. Secondly, building upon this foundation potential drug and hyperbaric oxygen effects as cited in the medical literature will be offered both as a narrative and as a graphic table to accentuate these effects. Finally, pharmacological precautions and contraindications as they relate to drug use and hyperbaric oxygen therapy will be offered.
The purpose of this review is to offer clinicians information regarding oxygen drug and physiology effects within the context of HBOT, increased atmospheric pressure, and lower extremity wound care. In order to accomplish this endeavor, specific concepts must be exemplified. An overview describing human pharmacokinetics, observed human physiological effects of hyperbaric oxygenation and pressure will be first offered because achieving this understanding is essential when discussing both pharmacodynamic and pharmacokinetic principles of drug-drug interactions. Secondly, building upon this foundation potential drug and hyperbaric oxygen effects as cited in the medical literature will be offered both as a narrative and as a graphic table to accentuate these effects. Finally, pharmacological precautions and contraindications as they relate to drug use and hyperbaric oxygen therapy will be offered.
Review of Pharmacology Principles
Clinical healthcare providers should recall that pharmacology is the study of the interaction of chemicals with biological systems. The science of pharmacology encompasses both pharmacokinetics, which is the science that describes the body’s action on a medicinal agent, and pharmacodynamics, which is the scientific description of the medicinal agent’s action on the body’s systems. Pharmacokinetics involves four major body functions: absorption, distribution, metabolism, and excretion. Absorption is the rate at which and extent to which a drug leaves its site of administration. Drug absorption occurs at different sites along the gastrointestinal tract, including the stomach and the small and large intestines. Once absorbed, most drugs bind to plasma proteins that are specific for some aspect or structural feature of the drug. After a drug is absorbed or injected into the blood stream it enters the circulation and is distributed throughout the body. Drug distribution is the process by which a drug reversibly leaves the blood stream and enters the extracellular fluid or the cells of the tissues. Drugs can be distributed into different compartments of the body (ie, blood, plasma, fat, or bone). The term volume- of-distribution is commonly used to describe the extent of drug distribution to tissues relative to the plasma volume. Drug metabolism is a biochemical enzyme-mediated reaction resulting in structural modification to the drug that changes its biological activity and/or water solubility. Drug metabolism occurs as a result of enzymatic reactions on the medications resulting in metabolites that may be active or rendered inactive. Body organs such as the gastrointestinal wall, lungs, and the liver, as well as the blood possess enzymes that metabolize drugs.11–13 Metabolism via the smooth endoplasmic reticulum of the liver is the first step in the elimination of many drugs.11,13 Drug metabolism by the liver occurs through one or both biotransformation reactions classified as either Phase I or Phase II reactions.12 Phase I reactions modify the drug by using oxidation, hydrolysis, and reduction. Oxidation involves the enzymatic addition of oxygen or removal of hydrogen, carried out by mixed function oxidases, often in the liver. Oxidative reactions typically involve a cytochrome P450 monooxygenase, NADPH, and oxygen. These modifying reactions create a more polar and highly water-soluble drug molecule for elimination by the kidney. Phase II reactions modify the drug pharmacologically to an inactive form using conjugation resulting in glucuronides, acetates, and sulfates. This is accomplished by the formation of a covalent linkage between a functional group appearing on the parent drug as a result of phase I metabolism and endogenously derived glucuronic acid, sulfate, glutathione, amino acids, or acetate.11,14 This new drug metabolite may now be eliminated by the kidney.  Some important preventable drug interactions are due to their effects on drug metabolizing enzymes, resulting in either reduced activity of the enzyme or increased activity of the enzyme, referred to as enzyme induction. The major group of enzymes in the liver responsible for metabolizing drugs can be isolated in a sub-cellular fraction termed the “microsomes.” Cytochrome P450 is a superfamily of enzymes bound to the cell membrane that are the terminal oxidases of this oxidation system. “Cytochrome” means colored cells; these enzymes contain iron giving the liver its red color. “P450” comes from the observation that the enzyme absorbs a very characteristic wavelength (450 nm) of ultraviolet light when it is exposed to carbon monoxide. These enzymes are named according to families that are defined by the similarity of their amino acid sequence. The nomenclature of each cytochrome isoenzyme follows some simple rules.15,16 Using CYP3A4 as an example, root of its name is CYP. Its family is noted with the number 3, while its subfamily is represented with the letter A. Its gene is denoted with the last number in the series 4. These P450 iso-enzymes are denoted with the following numbers and letters: “CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4.”15–17 More than 50% of currently used medications that are metabolized undergo CYP3A4 metabolism. The CYP3A subfamily is of particular interest because it is responsible for the metabolism of a large number of clinically important drugs in humans.16 The CYP3A4 isozyme accounts for over 25% of hepatic CYP450 content, and is responsible for more than half of all CYP450-mediated drug metabolism. Close to 14% of the adult liver contains a substantial proportion of CYP3A5, but it is proportionally more important in intestinal tissue and is the primary CYP3A enzyme in the kidney 16,17 Metabolism and elimination are responsible either separately or together for drug inactivation. Without these two pharmacokinetic functions, drugs would continuously circulate through the body interacting with various body receptors and interrupting important physiological processes.18 Drugs are either eliminated directly or converted into metabolites that are subsequently excreted. Removal of a drug from the body may occur by a number of routes, the most important being through the kidney into the urine. Other routes of elimination for drugs from the body include sweat, tears, breast milk, or expired air.
Some important preventable drug interactions are due to their effects on drug metabolizing enzymes, resulting in either reduced activity of the enzyme or increased activity of the enzyme, referred to as enzyme induction. The major group of enzymes in the liver responsible for metabolizing drugs can be isolated in a sub-cellular fraction termed the “microsomes.” Cytochrome P450 is a superfamily of enzymes bound to the cell membrane that are the terminal oxidases of this oxidation system. “Cytochrome” means colored cells; these enzymes contain iron giving the liver its red color. “P450” comes from the observation that the enzyme absorbs a very characteristic wavelength (450 nm) of ultraviolet light when it is exposed to carbon monoxide. These enzymes are named according to families that are defined by the similarity of their amino acid sequence. The nomenclature of each cytochrome isoenzyme follows some simple rules.15,16 Using CYP3A4 as an example, root of its name is CYP. Its family is noted with the number 3, while its subfamily is represented with the letter A. Its gene is denoted with the last number in the series 4. These P450 iso-enzymes are denoted with the following numbers and letters: “CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4.”15–17 More than 50% of currently used medications that are metabolized undergo CYP3A4 metabolism. The CYP3A subfamily is of particular interest because it is responsible for the metabolism of a large number of clinically important drugs in humans.16 The CYP3A4 isozyme accounts for over 25% of hepatic CYP450 content, and is responsible for more than half of all CYP450-mediated drug metabolism. Close to 14% of the adult liver contains a substantial proportion of CYP3A5, but it is proportionally more important in intestinal tissue and is the primary CYP3A enzyme in the kidney 16,17 Metabolism and elimination are responsible either separately or together for drug inactivation. Without these two pharmacokinetic functions, drugs would continuously circulate through the body interacting with various body receptors and interrupting important physiological processes.18 Drugs are either eliminated directly or converted into metabolites that are subsequently excreted. Removal of a drug from the body may occur by a number of routes, the most important being through the kidney into the urine. Other routes of elimination for drugs from the body include sweat, tears, breast milk, or expired air.
Hyperbaric Oxygenation and Pressure on Human Physiology
The hyperbaric environment is associated with physiological changes in the central nervous system, the endocrine system, respiration and hemodynamics.19 Hyperbaric oxygen reduces cerebral edema and improves the function of neurons damaged by ischemia or hypoxia. Changes in various endocrine organs have been reported under hyperbaric oxygenation. A fall in blood glucose has been observed in volunteers exposed to hyperbaric oxygen. However, there is scant data describing basic experimental studies assessing the effect of hyperbaric oxygen in experimentally induced diabetes mellitus. Patients with diabetes who are given hyperbaric oxygen for other indications should be carefully monitored for changes in blood glucose, as the insulin requirements are usually reduced and the dosage needs to be readjusted. Dreval et al20 devised a calculation method to assess the effects of hyperbaric oxygen in lowering the insulin requirement in these patients. Hyperoxia suppresses the respiratory reactivity to CO2. Hyperbaric oxygen reversibly depresses the hypoxic ventilatory drive by a direct effect on the carotid CO2 chemoreceptors. Hyperbaric oxygen therapy has a limited role in pulmonary disorders. Usually there are no differences between forced vital capacities and maximal expiratory flows before and after hyperbaric oxygen exposure while breathing dry or humidified oxygen.21 In human patients, hyperbaric oxygen defined as 100% oxygen results in a decrease in cardiac output by 10%–20% due to heart rate reduction rather than a reduction in stroke volume.19,21 Animal experiments reveal evidence that hyperbaric and hyperoxic conditions cause changes in cardiac output distribution.19,22,23 Risberg et al23 observational experiments demonstrated that the perfusion of several organs was markedly affected. Arterial liver perfusion was significantly increased while kidney and spleen perfusion were significantly reduced after 75 minutes but not after 15 minutes at 5 bar depth.23 Blood pressure remains essentially unchanged. Blood flow to most organs falls in proportion to the fall of cardiac output except to the right and left ventricle of the heart.21 Vasoconstriction may be viewed as a regulatory mechanism to protect the healthy organs from exposure to excessive pO2. Hyperbaric oxygen improves the elasticity of the red blood cells and reduces platelet aggregation.21 Increases in partial pressure of oxygen in the blood disturbs the reduction of oxyhemoglobin causing an increase in solubility of CO2, thus there is a retention of CO2 leading to a slight rise of H+ ions in the tissues. Hyperbaric oxygenation therapy reduces excess lactate production in hypoxic states.21 The presence of hyperbaric oxygen affects mono-oxygenases pathways, and results in both biochemical and human physiological alterations in metabolic pathways. The biochemical effects of hyperbaric oxygen include cyclo-oxygenase inactivation resulting in decreased production of prostacyclin by hyperoxic tissues.21 Hyperoxia inhibits phenylalanine and tyrosine hydroxylase. Increased oxygen saturation of Tyrosine Hydroxylase leads to increased turnover of catecholamines.21 Both Succinic Dehydrogenase and Cytochrome Oxidase are activated by hyperbaric oxygen. Cytochrome Oxidase is involved in the cytochrome P450 monooxygenase system. In the vernacular of chemistry, “activated” means to accelerate a reaction. In the setting of biology, “activated” means to convert into biologically active derivatives. When applying both these relative definitions of “activated” to the science of pharmacokinetics specifically drug metabolism in the presence of hyperbaric oxygen, the inference that drug metabolism by the cytochrome P450 monooxygenase system is accelerated is a reasonable deduction, despite the lack of actual clinical evidence gathered from randomized control trials.
The presence of hyperbaric oxygen affects mono-oxygenases pathways, and results in both biochemical and human physiological alterations in metabolic pathways. The biochemical effects of hyperbaric oxygen include cyclo-oxygenase inactivation resulting in decreased production of prostacyclin by hyperoxic tissues.21 Hyperoxia inhibits phenylalanine and tyrosine hydroxylase. Increased oxygen saturation of Tyrosine Hydroxylase leads to increased turnover of catecholamines.21 Both Succinic Dehydrogenase and Cytochrome Oxidase are activated by hyperbaric oxygen. Cytochrome Oxidase is involved in the cytochrome P450 monooxygenase system. In the vernacular of chemistry, “activated” means to accelerate a reaction. In the setting of biology, “activated” means to convert into biologically active derivatives. When applying both these relative definitions of “activated” to the science of pharmacokinetics specifically drug metabolism in the presence of hyperbaric oxygen, the inference that drug metabolism by the cytochrome P450 monooxygenase system is accelerated is a reasonable deduction, despite the lack of actual clinical evidence gathered from randomized control trials.
Hyperbaric Oxygenation and Pressure Effects on Medications
Oxygen is one of the most widely used pharmacological agents. When oxygen is breathed in concentrations higher than those in the atmospheric air, it is considered to be a drug. Under elevated partial pressure oxygen behaves like any other drug; too little is ineffective and too much will cause harm. It is important for the clinician to realize that oxygen under a hyperbaric pressure environment can interact with other drugs. Either potentiate or reduce the effects of other medications. Medications in a patient’s drug regimen taken for chronic illnesses may either reduce or potentiate the effects of hyperbaric oxygen. Also, many medications (including nonprescription medications) have undesirable side effects that may be modified in a hyperbaric oxygen environment. The distribution of a drug is affected by multiple body composition parameters: plasma volume, body mass index, average blood flow, total body water, plasma proteins, body fat, and cardiac output.18 Both liver blood flow and hepatic enzyme activity influence hepatic clearance of drugs. Because the perfusion of organs responsible for drug absorption or elimination may be altered, pharmacokinetics may also change under hyperbaric conditions.19 No study was found among the available medical compendium describing the influence of hyperbaria or hyperoxia on drug absorption from the gut. However, applying the observation that drug absorption is known to be altered in patients with congestive heart failure, it is plausible to make the assertion that hyperbaric and hyperoxia environments may affect drug absorption due to reduced cardiac output. Liver perfusion is a major determinant of the clearance of drugs that demonstrate a high hepatic extraction rate from the plasma.19,21 Similarly, a reduction of renal perfusion and glomerular filtration may reduce the clearance of drugs eliminated through the kidney,19,21 thus alterations in both liver and kidney blood flow, as well as resulting changes in drug clearance under hyperbaric or hyperoxic conditions, may be expected due to environmentally induced hemodynamic changes.19,21 The efficiency of pharmacological agents is affected by pressure usually manifested as it decreases in activity.24 Drugs that act on cellular membranes include anesthetics, narcotics, and tranquilizers. These medicines are all affected by an increase in atmospheric pressure changes that result in observed decreased effectness.24 Recalling that the major enzymes involved in drug metabolism are the cytochrome P450 monooxygenases bound to cellular membranes allows for an inference that alterations of atmospheric pressure may interfere with a number of drug metabolism pathways. The generation of oxygen free radicals related to high pO2 values affect the permeability of biological membranes.19,21 Oxygen free radicals are highly reactive, toxic entities that induce lipid peroxidation and may alter both proteins and nucleic acids. Alterations in drug protein-binding rates are associated with quantitative or qualitative changes in proteins. Unfortunately, experimental clinical based evidence is not available on effects of hyperbaria or hyperoxia environmental alterations on the protein-binding rates of drugs. Theoretically, one can infer that drugs displaying a high protein-binding rate should be most affected, demonstrating an altered volume of distribution and altered drug clearance because of the generation of oxygen free radicals. Further, the generation of oxygen free radicals might affect the activity of drug-metabolizing enzymes that affect hepatic clearance of drugs with a capacity-limited hepatic elimination.19  The medical community appreciates that it is impossible to remember all possible drug interactions. Conversely, a physician may apply an understanding of the real and theoretical mechanisms of drug interactions between hyperbaric oxygen, pressure, and drugs to empower themselves to avoid major adverse events caused by drug interactions. Advances in the understanding of the cytochrome monooxygenases system have made it possible to associate specific enzyme activity with the formation of a particular metabolite and, in some cases, to identify the major isoenzyme responsible for total clearance of a drug. Data regarding interactions between medications and hyperbaric oxygen from the scientific literature were identified by searching current compendia using both the electronic source (PubMed), as well as manual searches of bibliographies and reference listings.19,25–67 Figure 1 delineates the search criteria scheme graphically. Three hundred and forty-seven (347) citations were found and evaluated as defined by search limitations and Medical Subject Headings (ie, MeSH terms) for the search dates 1962–2010. These MeSH terms included: “hyperbaric oxygen,” “HBOT,” “drugs,” “the specific pharmacologic agent’s generic name,” “pharmacology,” and “interactions.” One hundred and seventeen (117, 34%) were found to be relevant for this review. Forty-three citations are included in this review. The majority of citations (n = 17) are defined as case reports or case studies by their authors. Observations and data from nine randomized control trials were assessed as well as observations and data from eight animal studies or laboratory experiments as it related specifically identified pharmacological generic names. The remaining citations were either author reviews or consensus from noted experts of hyperbaric medicine. For this review, observed pharmacokinetic and pharmacodynamic changes resulting from the interaction with hyperbaric oxygen was applied to the 100 most frequently prescribed medications in 2009 (Table 1).
The medical community appreciates that it is impossible to remember all possible drug interactions. Conversely, a physician may apply an understanding of the real and theoretical mechanisms of drug interactions between hyperbaric oxygen, pressure, and drugs to empower themselves to avoid major adverse events caused by drug interactions. Advances in the understanding of the cytochrome monooxygenases system have made it possible to associate specific enzyme activity with the formation of a particular metabolite and, in some cases, to identify the major isoenzyme responsible for total clearance of a drug. Data regarding interactions between medications and hyperbaric oxygen from the scientific literature were identified by searching current compendia using both the electronic source (PubMed), as well as manual searches of bibliographies and reference listings.19,25–67 Figure 1 delineates the search criteria scheme graphically. Three hundred and forty-seven (347) citations were found and evaluated as defined by search limitations and Medical Subject Headings (ie, MeSH terms) for the search dates 1962–2010. These MeSH terms included: “hyperbaric oxygen,” “HBOT,” “drugs,” “the specific pharmacologic agent’s generic name,” “pharmacology,” and “interactions.” One hundred and seventeen (117, 34%) were found to be relevant for this review. Forty-three citations are included in this review. The majority of citations (n = 17) are defined as case reports or case studies by their authors. Observations and data from nine randomized control trials were assessed as well as observations and data from eight animal studies or laboratory experiments as it related specifically identified pharmacological generic names. The remaining citations were either author reviews or consensus from noted experts of hyperbaric medicine. For this review, observed pharmacokinetic and pharmacodynamic changes resulting from the interaction with hyperbaric oxygen was applied to the 100 most frequently prescribed medications in 2009 (Table 1). 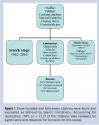 There is only a relatively small volume of data concerning observed pharmacokinetic studies regarding intravenous anesthetics and analgesics regarding the hyperbaric oxygen environment. Pentobarbital and merperidine have been investigated and showed no significant differences in half life, volume, and distribution, or plasma clearance at measured 2.8 and 6 atmospheres absolute (ATA).25 Camporesi26 recommends the use of intravenous sedation in the hyperbaric environment describing the use of ketamine and a benzodiazepine, along with a muscle relaxant, because these agents have proven to be very useful for induction and maintenance of anesthesia under hyperbaric conditions. Further, Camporesi26 stresses that while delivering anesthesia under hyperbaric conditions attention to detail is paramount. It should be delivered to be effective at pressure and not with any preconceived formulations.26 Narcotic analgesics generally depress respiration by reducing the reactivity to carbon dioxide in the medullary centers. Theoretically, combining the observed respiratory depressing effects of hyperbaric oxygen and the drug-induced depression observed with narcotic analgesics, the clinician must consider that a potential for an elevation in physiological paCO2 causing vasodilation and the potential for oxygen toxicity does exist when using narcotic analgesia and hyperbaric oxygen therapy. Beubler et al33 report on the practical aspects and the clinical application of oxygen under pressure using both mice and rats. These investigators report the analgesic effect of morphine was observed to be unchanged by the clinical application of oxygen under high pressure.33 Jain25 also reports the action of morphine is unchanged by hyperbaric oxygen. Conversely, central nervous stimulants such as amphetamines or related compounds such as methylphenidate may interact unfavorably with hyperbaric oxygen and may predispose the patient to oxygen toxicity. Rump et al review the influence of hyperbaric hyperoxia on the pharmacokinetics of lidocaine in two healthy men as a crossover trial experiment type at 1 and 2.5 bar.19 Lidocaine was given intravenously and blood samples were serially collected for 75 minutes.19 Lidocaine was measured by using an immunoassay and serum-concentration curves following an open two-compartment model.19 The pharmacokinetics parameters of lidocaine as recognized in the literature affirm its clearance from the body depends on liver plasma flow. Despite the fact that there are alterations in liver perfusion under hyperbaric or hyperoxic conditions, the results of this case study demonstrated that lidocaine disposition was not influenced by hyperbaric oxygen therapy.19 Using this observation these authors conclude that changes in liver perfusion at 2.5 bar breathing 100% O2 does not affect the clearance of drugs eliminated by hepatic plasma flow considered to be perfusion limited.19 The effect of hyperbaric oxygenation in anaerobic infections is well recognized. Oxygen acts as an antibiotic by impairing the bacterial metabolism. Hyperbaric oxygen is most effective in anaerobic infections. The nature of hyperbaric oxygen lends itself to be non-selective covering a broad spectrum of gram positive as well as gram-negative organisms. A state of hypoxia impairs the body’s immune system. The process of phagocytosis is impaired by hypoxia and improved with hyperbaric oxygen. Knighton et al28 published research that has demonstrated that breathing 45% oxygen is as effective as ampicillin in controlling certain aerobic bacterial inoculations, by stimulating leukocyte function. Oxygen may enhance the effects of other antimicrobial agents, particularly para-aminobenzoic antagonists, such as sulfonamides. The synergistic effects of combining hyperbaric oxygen at 2.87 ATA with sulfonamides (specifically sulfisoxazole) transforms its sole bacteriostatic action into more of a combined bactericidal mechanism of action.27 Efficacy of certain antibiotics, to include extended spectrum penicillin, aminoglycosides, vancomycin, and clindamycin, have been shown to be enhanced with hyperbaric oxygen without increasing antibiotic toxicity.29 Suggested mechanisms for the synergetic relationship between hyperbaric oxygen and antibiotics is described in one reference.29 First the increased pressure of oxygen in ischemic tissues improve the activity of aminoglycosides, fluoroquinolones, vancomycin, trimethoprim, and some sulfoamides.29 Secondly, inhibition of some reactions involved in bacterial biosynthesis, such as the enhancement of sulfonamide activity and the increased duration of post antibiotic effect of aminoglycosides in Pseudomonas infections.29 Finally, altered Redox potential of the bacteria, combined with an increase in reactive intermediates as in nitrofurantoin that requires a low Redox potential.29 Merritt and Slade30 investigated the influence of hyperbaric oxygen on the pharmacokinetics of single-dose of gentamicin in five healthy men between 28- and 43-years-old in a randomized crossover trial. Gentamicin disposition was studied under normobaric control conditions and under hyperbaric and hyperoxic conditions.30 Gentamicin doses of 1.5 mg/kg of lean body weight were intravenously infused in 100 mL over 30 minutes. Blood samples totaling eleven were serially collected over 300 minutes.30 Gentamicin clearance depends on glomerular filtration rate, which is reflected by creatinine clearance. Despite observations that hyperbaric oxygenation has shown to decrease renal blood flow,21,31 data from this investigation demonstrated that exposure to 2.4 ATA for 90 minutes did not alter gentamicin in a clinical manner.30 Clinical practice does recommend that Mafenide (Sulfamylon®) a topical antibacterial agent used in burn patients, be removed from all surfaces before they undergo hyperbaric oxygenation therapy.25 Mafenide is classified pharmacologically as a carbonic anhydrase inhibitor. It promotes CO2 retention and vasodilatation when placed in a hyperbaric oxygen environment. Acetazolamide, another carbonic anhydrase inhibitor used to treat moderate to severe metabolic alkalosis as well as glaucoma, epileptic seizures, benign intracranial hypertension (pseudotumor cerebri), altitude sickness, cystinuria, and dural ectasia prevents oxygen-induced vasoconstriction and increases blood flow in the presence of hyperbaric oxygenation. Therefore it may predispose the brain to oxygen toxicity.25 Wood32 used animal studies to demonstrate acetazolamide and 5% CO2 shorten time to onset of convulsions and suggest that increased tissue levels of CO2 play an important role in hyperbaric oxygen toxicity. Bove34 relates the premise that there is a very distinct possibility that patients selected to undergo hyperbaric oxygen therapy will have one or more pharmacological therapeutic agents classified as cardiovascular medications. Moreover, Bove recommends that cardiovascular medications can be continued during hyperbaric oxygen therapy; however, these agents should be reviewed in the context of the presenting current medical condition.34 Further, Jain25 offers the observation that the hypotensive effects of both alpha and beta-blockers, ganglion blockers, and β-adrenomimetics are considerably reduced in the hyperbaric oxygen environment. Other observations include both the direct and indirect “pressor” effects of α-adrenomimetics, as well as the cardiotropic effects of β-adrenoblockers are potentiated.25 Filatov and Reznikov35 related observations in rats suggesting that the action of oxygen is mediated via its direct, but primarily indirect action of as adrenomimetic and beta adreno-blocking effects. Given these observations, Jain25 recommends that these drugs should be given after and not before a scheduled hyperbaric oxygen session. The influence of hyperbaric oxygen and pressure has been extensively studied in propranolol in both animals and humans.36–39 The influence of both beta sympathetic agonists and blocking agents on oxygen at high pressure toxicity was examined in rats by Crittenden and Beckman.36 Rats were exposed oxygen at high pressure (6 ATA) and examined for time to seizures. Pretreated propranolol rats demonstrated a 70% increase in time to seizure, as well as prevention of brain depletion of glycogen prior to seizure.36 Their results suggest a possible role for secondary messengers mediating some of the acute CNS toxic effects of oxygen at high pressure toxicity, because propranolol blocks the beta-receptor influence on adenyl cyclase-simulated second messenger production.36 Torbati37 measured the effect of 1, 2, and 5 mg/kg of propranolol on the neuroelectrophysiological manifestations of CNS oxygen toxicity in conscious rats. The results of this research suggest that the protective effect of propranolol against the neurological manifestations of oxygen toxicity may be related to propranolol’s multiple effects on physiological systems rather than beta adrenergic blocking action alone.37 The positive clinical effect of hyperbaric oxygen therapy has been shown to be accompanied by higher activity of superoxide dimutase in red blood cells.38 This enzyme catalyzes the conversion of superoxide into oxygen and hydrogen peroxide. Oxygen free radicals are normally removed in our bodies by the superoxide dismutase enzyme, which functions as an antioxidant and anti-inflammatory to protect the human body from harm. The combination of propranolol (40 mg) and a 40-minute hyperbaric oxygenation session at 1.5 ATA was studied in 33 men with coronary heart disease.39 These investigators concluded propranolol induced a supplemental reduction in sympathetic activity causing the drug’s negative chronotropic and inotropic effects to be potentiated.39,40 Al- Waili et al41 offer salient advice that the use of beta blockers for the management of hypertension should be avoided during hyperbaric oxygen therapy, because their results showed that the use of beta-blockers in 41 patients with hypertension and diabetes mellitus caused a significant elevation of blood pressure while reducing heart rate.41 Other pharmacological agents that may be therapeutically beneficial in cardiovascular or circulatory pathologies that have been studied in combination with hyperbaric oxygen under include: nitroglycerin (depot-glycerol trinitrate), nifedipine, aspirin, pentoxifylline, digoxin, heparin, enoxaparin, and losartan.38–40,42–51 Seriakov et al40 demonstrated while studying 35 patients with ischemic heart disease that a single hyperbaric oxygenation session lasting 40 minutes at a pressure of 1.5 atmosphere had no impact on the degree of hemodynamic effect of depot-glycerol trinitrate. Intravenous nitroglycerin has been administered to animals in an effort to prevent bubble formation during decompression sickness.42 In the animals that received NTG at 0.4 mcg/kg/min for 30 minutes significantly fewer bubbles were detected after decompression and no deaths resulted.42 The indirect hemodynamic effect of nifedipine has been shown to be reduced during a hyperbaric oxygenation session.40 Despite animal studies25 that revealed that salicylate clearance was significantly increased at an atmosphere of 2.8, Seriakov and Feofanova43 concluded that the platelet anti-aggregant activity of aspirin at a daily dose of 125 mg or pentoxifylline dose at 300 mg daily was not altered during 8 to 12 hyperbaric sessions lasting 40 minutes at depths of 1.3–1.6 ATA. A recent case report describing the treatment of retinal artery embolization during carotid angioplasty and carotid artery stenting validates the concurrent use of aspirin, ticlopidine, and hyperbaric oxygen for 1 week without ill effects to the patient.44 Animal research has produced findings suggesting that the effectiveness of cardiac glycosides (Digitalis/Digoxin) was decreased in the presence of 100% hyperbaric oxygen sessions.45 Initial results from animal experimentation showed that heparin treated animals exposed to hyperbaric oxygen developed pulmonary hemorrhages.46 It was concluded that the heparin-hyperoxic interaction during development of pulmonary and CNS oxygen toxicity may be related to the anticoagulant effect of heparin and hyperoxic-induced pulmonary lesions.46 Quite the opposite results are reported in three recent case reports describing the successful use of either heparin or low-molecular-weight heparin in combination of hyperbaric oxygen without ill effects to patients.47–49 One case report discusses the beneficial effects of systemic heparin and hyperbaric oxygen therapy to prevent liver failure in an infant who underwent an orthotopic liver transplant.46 Another case report presents information regarding successful use of a heparin infusion (1000 units/h) with concurrent hyperbaric oxygen therapy for 9 days to treat a 37-year-old man with frostbite of his right hand. The combination of heparin and hyperbaric oxygen therapy did not produce any adverse effects noted during the combination therapy.48 The last case report describes a 63-year-old Polynesian woman presenting with atypical calciphylaxis attributed to warfarin therapy.49 A therapeutic dose of enoxaparin was substituted for warfarin and the patient underwent forty sessions of hyperbaric oxygen sessions with no adverse effects noted and resolution of cutaneous lesions.49 Animal models have demonstrated that the combination of losartan, an angiotensin II antagonist, and the combination of hyperbaric oxygen therapy increases the drug’s efficacy and has significant benefits as it relates to the management of proteinuria.50,51 Human case control investigations have demonstrated hyperbaric oxygen therapy sessions improve metabolic control and reduce insulin requirements in patients with type 2 diabetes mellitus.41,52,53 Al-Waili et al41 revealed hyperbaric oxygen therapy sessions lowered blood glucose levels by 23% (P < 0.001). When the basal blood glucose level was between 120 mg/dL–170 mg/dL, it dropped to less than 100 mg/dL in 31/60 (52%) treatment sessions.41 When the basal blood glucose level was less than 120 mg/dL, it dropped to less than 70 mg/dL in 8/34 (23.5%) sessions.41 The combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetic patients to determine improvement of islet function and metabolic control was investigated by Estrada et al.52 Significant improvement was seen when comparing base line values and 12-month follow up values of fasting glucose 205.6 ± 5.9 vs. 105.2 ± 14.2 mg/dL and HbA1c 8.8% ± 0.2 vs. 6.0% ± 0.4.49 Karadurmus et al’s recent investigation yielded similar gradual reduced results from base line values in fasting blood glucose and HBA1c after hyperbaric oxygen therapy ( FBG 152 ± 3.7 mg/dL vs. 113 ± 14 and HBA1c 9.1 ± 1.3 versus 8.0 ± 1.1).53 Given all these results collectively, the dosage of insulin among patients with diabetes during hyperbaric oxygen therapy sessions should be readjusted. One attractive metabolic effect of hyperbaric oxygen therapy is that it reduces excess lactate production in hypoxic states, as well as during exercise.21 This detail was appealing when evaluating a patient for hyperbaric oxygen therapy whose drug regimen may have included metformin, that is thought to increase the risk of lactic acidosis. Recently, pooled data from 347 comparative trials and cohort studies revealed no cases of fatal or nonfatal lactic acidosis in 70,490 patient years of metformin use.54 These authors found no evidence from prospective comparative trials or from observational cohorts that metformin is associated with an increase risk of lactic acidosis, or increased levels of lactate, compared to other anti-hyperglycemic treatments.54 Finally, despite the lack of actual clinical based evidence, the clinical results observed during hyperbaric oxygen therapy sessions on blood glucose levels among patients with diabetes may be applied to other pharmacologic oral agents with hypoglycemic actions like thiazolidinediones, sulfonylureas, and perhaps sitaglipyin (DPP-4 inhibitor). Two medications that may enhance oxygen toxicity and must be used with caution when electing HBOT, besides acetazolamide, includes thyroid extract and disulfiram (Antabuse®), which is used in alcohol aversion therapy. It may potentiate oxygen toxicity through in-vivo reduction to diethyldithiocarbamate and subsequent inhibition of superoxide dismutase.25 Animal experiments have revealed that those animals given thyroid or thyroid extract under hyperbaric oxygen conditions have an enhanced chance of experiencing oxygen toxicity. The drug induced increase in metabolic rate is thought to predispose the subjects to oxygen-induced convulsions.25 It is not out of the realm of possibility to apply this data to humans and assume that the same adverse outcome could occur.
There is only a relatively small volume of data concerning observed pharmacokinetic studies regarding intravenous anesthetics and analgesics regarding the hyperbaric oxygen environment. Pentobarbital and merperidine have been investigated and showed no significant differences in half life, volume, and distribution, or plasma clearance at measured 2.8 and 6 atmospheres absolute (ATA).25 Camporesi26 recommends the use of intravenous sedation in the hyperbaric environment describing the use of ketamine and a benzodiazepine, along with a muscle relaxant, because these agents have proven to be very useful for induction and maintenance of anesthesia under hyperbaric conditions. Further, Camporesi26 stresses that while delivering anesthesia under hyperbaric conditions attention to detail is paramount. It should be delivered to be effective at pressure and not with any preconceived formulations.26 Narcotic analgesics generally depress respiration by reducing the reactivity to carbon dioxide in the medullary centers. Theoretically, combining the observed respiratory depressing effects of hyperbaric oxygen and the drug-induced depression observed with narcotic analgesics, the clinician must consider that a potential for an elevation in physiological paCO2 causing vasodilation and the potential for oxygen toxicity does exist when using narcotic analgesia and hyperbaric oxygen therapy. Beubler et al33 report on the practical aspects and the clinical application of oxygen under pressure using both mice and rats. These investigators report the analgesic effect of morphine was observed to be unchanged by the clinical application of oxygen under high pressure.33 Jain25 also reports the action of morphine is unchanged by hyperbaric oxygen. Conversely, central nervous stimulants such as amphetamines or related compounds such as methylphenidate may interact unfavorably with hyperbaric oxygen and may predispose the patient to oxygen toxicity. Rump et al review the influence of hyperbaric hyperoxia on the pharmacokinetics of lidocaine in two healthy men as a crossover trial experiment type at 1 and 2.5 bar.19 Lidocaine was given intravenously and blood samples were serially collected for 75 minutes.19 Lidocaine was measured by using an immunoassay and serum-concentration curves following an open two-compartment model.19 The pharmacokinetics parameters of lidocaine as recognized in the literature affirm its clearance from the body depends on liver plasma flow. Despite the fact that there are alterations in liver perfusion under hyperbaric or hyperoxic conditions, the results of this case study demonstrated that lidocaine disposition was not influenced by hyperbaric oxygen therapy.19 Using this observation these authors conclude that changes in liver perfusion at 2.5 bar breathing 100% O2 does not affect the clearance of drugs eliminated by hepatic plasma flow considered to be perfusion limited.19 The effect of hyperbaric oxygenation in anaerobic infections is well recognized. Oxygen acts as an antibiotic by impairing the bacterial metabolism. Hyperbaric oxygen is most effective in anaerobic infections. The nature of hyperbaric oxygen lends itself to be non-selective covering a broad spectrum of gram positive as well as gram-negative organisms. A state of hypoxia impairs the body’s immune system. The process of phagocytosis is impaired by hypoxia and improved with hyperbaric oxygen. Knighton et al28 published research that has demonstrated that breathing 45% oxygen is as effective as ampicillin in controlling certain aerobic bacterial inoculations, by stimulating leukocyte function. Oxygen may enhance the effects of other antimicrobial agents, particularly para-aminobenzoic antagonists, such as sulfonamides. The synergistic effects of combining hyperbaric oxygen at 2.87 ATA with sulfonamides (specifically sulfisoxazole) transforms its sole bacteriostatic action into more of a combined bactericidal mechanism of action.27 Efficacy of certain antibiotics, to include extended spectrum penicillin, aminoglycosides, vancomycin, and clindamycin, have been shown to be enhanced with hyperbaric oxygen without increasing antibiotic toxicity.29 Suggested mechanisms for the synergetic relationship between hyperbaric oxygen and antibiotics is described in one reference.29 First the increased pressure of oxygen in ischemic tissues improve the activity of aminoglycosides, fluoroquinolones, vancomycin, trimethoprim, and some sulfoamides.29 Secondly, inhibition of some reactions involved in bacterial biosynthesis, such as the enhancement of sulfonamide activity and the increased duration of post antibiotic effect of aminoglycosides in Pseudomonas infections.29 Finally, altered Redox potential of the bacteria, combined with an increase in reactive intermediates as in nitrofurantoin that requires a low Redox potential.29 Merritt and Slade30 investigated the influence of hyperbaric oxygen on the pharmacokinetics of single-dose of gentamicin in five healthy men between 28- and 43-years-old in a randomized crossover trial. Gentamicin disposition was studied under normobaric control conditions and under hyperbaric and hyperoxic conditions.30 Gentamicin doses of 1.5 mg/kg of lean body weight were intravenously infused in 100 mL over 30 minutes. Blood samples totaling eleven were serially collected over 300 minutes.30 Gentamicin clearance depends on glomerular filtration rate, which is reflected by creatinine clearance. Despite observations that hyperbaric oxygenation has shown to decrease renal blood flow,21,31 data from this investigation demonstrated that exposure to 2.4 ATA for 90 minutes did not alter gentamicin in a clinical manner.30 Clinical practice does recommend that Mafenide (Sulfamylon®) a topical antibacterial agent used in burn patients, be removed from all surfaces before they undergo hyperbaric oxygenation therapy.25 Mafenide is classified pharmacologically as a carbonic anhydrase inhibitor. It promotes CO2 retention and vasodilatation when placed in a hyperbaric oxygen environment. Acetazolamide, another carbonic anhydrase inhibitor used to treat moderate to severe metabolic alkalosis as well as glaucoma, epileptic seizures, benign intracranial hypertension (pseudotumor cerebri), altitude sickness, cystinuria, and dural ectasia prevents oxygen-induced vasoconstriction and increases blood flow in the presence of hyperbaric oxygenation. Therefore it may predispose the brain to oxygen toxicity.25 Wood32 used animal studies to demonstrate acetazolamide and 5% CO2 shorten time to onset of convulsions and suggest that increased tissue levels of CO2 play an important role in hyperbaric oxygen toxicity. Bove34 relates the premise that there is a very distinct possibility that patients selected to undergo hyperbaric oxygen therapy will have one or more pharmacological therapeutic agents classified as cardiovascular medications. Moreover, Bove recommends that cardiovascular medications can be continued during hyperbaric oxygen therapy; however, these agents should be reviewed in the context of the presenting current medical condition.34 Further, Jain25 offers the observation that the hypotensive effects of both alpha and beta-blockers, ganglion blockers, and β-adrenomimetics are considerably reduced in the hyperbaric oxygen environment. Other observations include both the direct and indirect “pressor” effects of α-adrenomimetics, as well as the cardiotropic effects of β-adrenoblockers are potentiated.25 Filatov and Reznikov35 related observations in rats suggesting that the action of oxygen is mediated via its direct, but primarily indirect action of as adrenomimetic and beta adreno-blocking effects. Given these observations, Jain25 recommends that these drugs should be given after and not before a scheduled hyperbaric oxygen session. The influence of hyperbaric oxygen and pressure has been extensively studied in propranolol in both animals and humans.36–39 The influence of both beta sympathetic agonists and blocking agents on oxygen at high pressure toxicity was examined in rats by Crittenden and Beckman.36 Rats were exposed oxygen at high pressure (6 ATA) and examined for time to seizures. Pretreated propranolol rats demonstrated a 70% increase in time to seizure, as well as prevention of brain depletion of glycogen prior to seizure.36 Their results suggest a possible role for secondary messengers mediating some of the acute CNS toxic effects of oxygen at high pressure toxicity, because propranolol blocks the beta-receptor influence on adenyl cyclase-simulated second messenger production.36 Torbati37 measured the effect of 1, 2, and 5 mg/kg of propranolol on the neuroelectrophysiological manifestations of CNS oxygen toxicity in conscious rats. The results of this research suggest that the protective effect of propranolol against the neurological manifestations of oxygen toxicity may be related to propranolol’s multiple effects on physiological systems rather than beta adrenergic blocking action alone.37 The positive clinical effect of hyperbaric oxygen therapy has been shown to be accompanied by higher activity of superoxide dimutase in red blood cells.38 This enzyme catalyzes the conversion of superoxide into oxygen and hydrogen peroxide. Oxygen free radicals are normally removed in our bodies by the superoxide dismutase enzyme, which functions as an antioxidant and anti-inflammatory to protect the human body from harm. The combination of propranolol (40 mg) and a 40-minute hyperbaric oxygenation session at 1.5 ATA was studied in 33 men with coronary heart disease.39 These investigators concluded propranolol induced a supplemental reduction in sympathetic activity causing the drug’s negative chronotropic and inotropic effects to be potentiated.39,40 Al- Waili et al41 offer salient advice that the use of beta blockers for the management of hypertension should be avoided during hyperbaric oxygen therapy, because their results showed that the use of beta-blockers in 41 patients with hypertension and diabetes mellitus caused a significant elevation of blood pressure while reducing heart rate.41 Other pharmacological agents that may be therapeutically beneficial in cardiovascular or circulatory pathologies that have been studied in combination with hyperbaric oxygen under include: nitroglycerin (depot-glycerol trinitrate), nifedipine, aspirin, pentoxifylline, digoxin, heparin, enoxaparin, and losartan.38–40,42–51 Seriakov et al40 demonstrated while studying 35 patients with ischemic heart disease that a single hyperbaric oxygenation session lasting 40 minutes at a pressure of 1.5 atmosphere had no impact on the degree of hemodynamic effect of depot-glycerol trinitrate. Intravenous nitroglycerin has been administered to animals in an effort to prevent bubble formation during decompression sickness.42 In the animals that received NTG at 0.4 mcg/kg/min for 30 minutes significantly fewer bubbles were detected after decompression and no deaths resulted.42 The indirect hemodynamic effect of nifedipine has been shown to be reduced during a hyperbaric oxygenation session.40 Despite animal studies25 that revealed that salicylate clearance was significantly increased at an atmosphere of 2.8, Seriakov and Feofanova43 concluded that the platelet anti-aggregant activity of aspirin at a daily dose of 125 mg or pentoxifylline dose at 300 mg daily was not altered during 8 to 12 hyperbaric sessions lasting 40 minutes at depths of 1.3–1.6 ATA. A recent case report describing the treatment of retinal artery embolization during carotid angioplasty and carotid artery stenting validates the concurrent use of aspirin, ticlopidine, and hyperbaric oxygen for 1 week without ill effects to the patient.44 Animal research has produced findings suggesting that the effectiveness of cardiac glycosides (Digitalis/Digoxin) was decreased in the presence of 100% hyperbaric oxygen sessions.45 Initial results from animal experimentation showed that heparin treated animals exposed to hyperbaric oxygen developed pulmonary hemorrhages.46 It was concluded that the heparin-hyperoxic interaction during development of pulmonary and CNS oxygen toxicity may be related to the anticoagulant effect of heparin and hyperoxic-induced pulmonary lesions.46 Quite the opposite results are reported in three recent case reports describing the successful use of either heparin or low-molecular-weight heparin in combination of hyperbaric oxygen without ill effects to patients.47–49 One case report discusses the beneficial effects of systemic heparin and hyperbaric oxygen therapy to prevent liver failure in an infant who underwent an orthotopic liver transplant.46 Another case report presents information regarding successful use of a heparin infusion (1000 units/h) with concurrent hyperbaric oxygen therapy for 9 days to treat a 37-year-old man with frostbite of his right hand. The combination of heparin and hyperbaric oxygen therapy did not produce any adverse effects noted during the combination therapy.48 The last case report describes a 63-year-old Polynesian woman presenting with atypical calciphylaxis attributed to warfarin therapy.49 A therapeutic dose of enoxaparin was substituted for warfarin and the patient underwent forty sessions of hyperbaric oxygen sessions with no adverse effects noted and resolution of cutaneous lesions.49 Animal models have demonstrated that the combination of losartan, an angiotensin II antagonist, and the combination of hyperbaric oxygen therapy increases the drug’s efficacy and has significant benefits as it relates to the management of proteinuria.50,51 Human case control investigations have demonstrated hyperbaric oxygen therapy sessions improve metabolic control and reduce insulin requirements in patients with type 2 diabetes mellitus.41,52,53 Al-Waili et al41 revealed hyperbaric oxygen therapy sessions lowered blood glucose levels by 23% (P < 0.001). When the basal blood glucose level was between 120 mg/dL–170 mg/dL, it dropped to less than 100 mg/dL in 31/60 (52%) treatment sessions.41 When the basal blood glucose level was less than 120 mg/dL, it dropped to less than 70 mg/dL in 8/34 (23.5%) sessions.41 The combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetic patients to determine improvement of islet function and metabolic control was investigated by Estrada et al.52 Significant improvement was seen when comparing base line values and 12-month follow up values of fasting glucose 205.6 ± 5.9 vs. 105.2 ± 14.2 mg/dL and HbA1c 8.8% ± 0.2 vs. 6.0% ± 0.4.49 Karadurmus et al’s recent investigation yielded similar gradual reduced results from base line values in fasting blood glucose and HBA1c after hyperbaric oxygen therapy ( FBG 152 ± 3.7 mg/dL vs. 113 ± 14 and HBA1c 9.1 ± 1.3 versus 8.0 ± 1.1).53 Given all these results collectively, the dosage of insulin among patients with diabetes during hyperbaric oxygen therapy sessions should be readjusted. One attractive metabolic effect of hyperbaric oxygen therapy is that it reduces excess lactate production in hypoxic states, as well as during exercise.21 This detail was appealing when evaluating a patient for hyperbaric oxygen therapy whose drug regimen may have included metformin, that is thought to increase the risk of lactic acidosis. Recently, pooled data from 347 comparative trials and cohort studies revealed no cases of fatal or nonfatal lactic acidosis in 70,490 patient years of metformin use.54 These authors found no evidence from prospective comparative trials or from observational cohorts that metformin is associated with an increase risk of lactic acidosis, or increased levels of lactate, compared to other anti-hyperglycemic treatments.54 Finally, despite the lack of actual clinical based evidence, the clinical results observed during hyperbaric oxygen therapy sessions on blood glucose levels among patients with diabetes may be applied to other pharmacologic oral agents with hypoglycemic actions like thiazolidinediones, sulfonylureas, and perhaps sitaglipyin (DPP-4 inhibitor). Two medications that may enhance oxygen toxicity and must be used with caution when electing HBOT, besides acetazolamide, includes thyroid extract and disulfiram (Antabuse®), which is used in alcohol aversion therapy. It may potentiate oxygen toxicity through in-vivo reduction to diethyldithiocarbamate and subsequent inhibition of superoxide dismutase.25 Animal experiments have revealed that those animals given thyroid or thyroid extract under hyperbaric oxygen conditions have an enhanced chance of experiencing oxygen toxicity. The drug induced increase in metabolic rate is thought to predispose the subjects to oxygen-induced convulsions.25 It is not out of the realm of possibility to apply this data to humans and assume that the same adverse outcome could occur.
Potential Interactions
The medications selected for review were the 100 most frequently prescribed (brand and generic names) in 2009 as measured by IMS Health based on $300.3 billion of prescriptions sold.55 A literature review was conducted to identify intrinsic drug interactions with hyperbaric oxygen. The data obtained were primarily qualitative and were based on reports in the current compendium, recent journal articles, and drug package inserts.25–67 Current literature sources were used to resolve conflicting information presented in reference materials. Information and drug interaction observations and recommendations presented in Table 1 are centered on the applied science of pharmacology and physiology, as well as clinical intuitive judgment balancing both aspects of patient benefit verses patient risk. The first limitation is that the majority of drug and hyperbaric oxygen interactions described do not rise to observations seen during high-quality randomized trials with statistically significant difference and a narrow confidence interval. The drug interaction observations seen with concurrent use of hyperbaric oxygen are either from case series or from expert opinion. Another limitation of the data presented in tertiary literature sources is that the data is based on animal studies that have to be interpreted and then applied to humans. A table was constructed listing 100 medications by either brand name or generic name, followed by the information concerning drug interactions (Table 1). During the review, it was noted duplicates were present owing to listing of medications by both brand name and generic name. Therefore, the actual number of different medications in the review was 69. Reported drug interactions resulting from the effects of hyperbaric oxygen occurred with 38 of the 69 drugs reviewed (55%). Descriptions of the possible effects of hyperbaric oxygen are presented for each reviewed medication. Thirty-one medications of the 69 that were reviewed (44.9%) did not have any description of the possible effects of hyperbaric oxygen. A few references recommended avoidance of hyperbaric oxygen because co-administration of these drugs predisposes the patient to oxygen toxicity. Possible drug interactions and the effects of hyperbaric oxygen on medications that were likely, but not certain to cause a negative interaction because they were only observed in animals or found in similar pharmacological agents or a parent drug, were noted as “probable.” 
Conclusion
Drug interactions with hyperbaric oxygen represent an important subject, but there is a lack of overwhelming clinical based evidence describing the effects of hyperbaric oxygen for many of the commonly prescribed medications. Clinicians should perform a careful patient medication history to avoid medications that may be adversely effected by hyperbaric oxygen. Therefore, this review offers the healthcare provider information regarding prescription drug interactions caused by hyperbaric oxygen. Mechanisms found throughout the literature of potential drug interactions caused by hyperbaric oxygen were presented. The 100 most frequently prescribed medications measured by IMS Health for 2009 were reviewed regarding hyperbaric oxygen as cited in the medical literature. The actual number of different medications review was 69. Reported drug interactions resulting from the effects of hyperbaric oxygen occurred with 38 of the 69 drugs reviewed (55%). Descriptions of the possible effects of hyperbaric oxygen were presented for each medication. Thirty-one medications of the 69 review drugs (44.9%) did not have any description of the possible effects of hyperbaric oxygen. Further, experimental research that rises to significant clinical evidence related to pharmacokinetic and pharmacodynamic interactions of commonly prescribed drugs receiving hyperbaric oxygen is recommended.
References
1. Prescription Drug Trends. Kaiser Family Foundations. September 2008. Available at: https://www.kff.org/rxdrugs/3057.cfm. 2. Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: US prescription drug data for 2007–2008. NCHS data brief. Hyattsville, MD: National Center for Health Statistics; 2010;42. 3. Bitterman H. Bench-to-bedside review: oxygen as a drug. Crit Care. 2009;13(1):205. 4. Gupta V, Vijay S, Gupta R, Koul S. Hyperbaric oxygen therapy. JK Pract. 2005;12(1):44–47. 5. Wunderlich RP, Peters EJ, Lavery LA. Systemic hyperbaric oxygen therapy: lower-extremity wound healing and the diabetic foot. Diabetes Care. 2000;23(10):1551–1555. 6. Wang C, Schwaitzberg S, Berliner E, Zarin DA, Lau J. Hyperbaric oxygen for treating wounds: a systematic review of the literature. Arch Surg. 2003;138(3):272–279. 7. Ong M. Hyperbaric oxygen therapy in management of diabetic lower limb wounds. Singapore Med J. 2008;49(2):105–109. 8. Kaya A, Aydin F, Altay T, et al. Can major amputation rates be decreased in diabetic foot ulcers with hyperbaric oxygen therapy? Int Orthop.2009;33(2):441–446. 9. Kranke P, Bennett M, Rocckl-Wiedman I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Databse Syst Rev. 2004;(2):CD004123. 10. Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33(5):998–1003. 11. Benet LZ, Kroetz DL, Sheiner LB. Pharmacokinetics: the dynamics of drug absorption, distribution, and elimination. In: Hardman JG, Limbird LE, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw Hill; 1996:3–27. 12. Bauer LA. Clinical Pharmacokinetics and pharmacodynamics In: Dipro JT, ed. Pharmacotherapy: A Pathophysiologic Approach. Stamford, CT: Appleton & Lange; 1999:21–30. 13. Hansten PD, Horn JR, eds. Drug interaction mechanisms: enzyme induction. In: Hansten and Horn’s Drug Interactions Analysis and Management. Freeland, WA: H&H Publications; 2003:PM1-PM-15. 14. Leucuta SE, Vlase L. Pharmacokinetics and metabolic drug interactions. Curr Clin Pharmacol. 2006;1(1):5–20. 15. Nelson DR, Koymans L, Kamataki T, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers, and nomenclature. Pharmacogenetics. 1996;6(1):1–42. 16. Streetman DS. Metabolic differences and pharmacogenetics: implications for anesthesia. Anesthesia Today. 2004;14:12. 17. Koch I, Weil R, Woldold R et al. Interindividual variability and tissue-specificity in the expression of cytochrome P450 3A mRNA. Drug Metab Dispos. 2002;30(10):1008–1114. 18. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinetics. 2009; 48(3):143–157. 19. Rump AF, Siekmann U, Kalff G. Effects of hyperbaric and hyperoxic conditions on the disposition of drugs: theoretical considerations and a review of the literature. Gen Pharmacol. 1999; 32(1):127–133. 20. Dreval AV, Dreval TP, Lukicheva, TI. Two-dimensional parameter of the kinetics of glucose (rho-criterion) in the assessment of the efficacy and prognosis of therapy of diabetes mellitus. Ter Arkh. 1988;60(9):20–24. 21. Jain KK. Physical, physiological, and biochemical aspects of hyperbaric oxygenation. In: Jain KK, ed. Textbook of Hyperbaric Medicine. 5th ed. Cambridge, MA: Hogrefe & Huber Publishers; 2009:9–19. 22. Hordnes C, Tyssebotn I. Effect of high ambient pressure and oxygen tension on organ blood flow in conscious trained rats. Undersea Biomed Res. 1985;12(2):115–128. 23. Risberg J. Bergø GW. Hordnes C, Tyssebotn I. Distribution of cardiac output in awake rats during exposure to 5 bar. Undersea Biomed Res. 1990;17(6):503–514. 24. Jain KK. Effects of diving and high pressure on the human body. In: Jain KK, ed. Textbook of Hyperbaric Medicine. 5th ed. Cambridge, MA: Hogrefe & Huber Publishers; 2009:21–29. 25. Jain KK. Drug Interactions with Hyperbaric Oxygen. In: Jain KK, ed. Textbook of Hyperbaric Medicine. 5th ed. Cambridge, MA: Hogrefe & Huber Publishers; 2009:81–84. 26. Camporesi EM. Anesthesia in the hyperbaric environment. In: Jain KK, ed. Textbook of Hyperbaric Medicine. 5th ed. Cambridge, MA: Hogrefe & Huber Publishers; 2009:447–452. 27. Jain KK. HBO therapy in infections. In: Jain KK, ed. Textbook of Hyperbaric Medicine. 5th ed. Cambridge, MA: Hogrefe & Huber Publishers; 2009:135–148. 28. Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic: a comparison of the effects of inspired oxygen concentration and antibiotic administration on in vivo bacterial clearance. Arch Surg. 1986;121(2):191–195. 29. Marzella L, Vezzani G. Effects of hyperbaric oxygen on activity of antibacterial agents. In: Oriani G, Marroni A, Waltel F, eds. Handbook of Hyperbaric Medicine. London: Springer; 1996:699–713. 30. Merritt GJ, Slade JB. Influence of hyperbaric oxygen on the pharmacokinetics of single-dose gentamicin in healthy volunteers. Pharmacotherapy. 1993;13(4):382–385. 31. Fischer B, Jain KK, Braun E, et al. Handbook of Hyperbaric Oxygen Therapy. New York, NY: Springer-Verlag; 1988:180–183. 32. Wood CD. Acetazolamide and CO2 in hyperbaric oxygen toxicity. Undersea Biomed Res. 1982; 9(1):15–20. 33. Beubler E, Lembeck F, Stolze A. Interactions between oxygen under pressure and drugs (author’s transl). Wien Klin Wochenschr. 1977;89(8):260–265. [Article in German]. 34. Bove AA. Cardiovascular aspects of hyperbaric oxygen therapy. In: Newman TS, Thom SR, eds. Physiology and Medicine of Hyperbaric Oxygen Therapy. Philadelphia, PA: Saunders Publishing; 2008:583. 35. Filatov AF, Reznikov KM. Effect of adrenomimetic, adreno- and ganglionic-blocking agents during hyperbaric oxygenation. Farmakol Toksikol. 1982;45(2):35–39. 36. Crittenden DJ, Beckman DL. Beta adrenergic receptors and glucagon in seizures from exposure to oxygen at high pressure (OHP). Life Sci. 1983;33(20):1959–1964. 37. Torbati D. Effect of propranolol on brain electrical activity during hyperbaric oxygenation in the rat. Undersea Biomed Res. 1985;12(4):423–429. 38. Seriakov VV, Konovalova GG, Sidorenko BA, et al. Hyperbaric oxygenation and antianginal agents: effects on blood levels of malanialdehyde and activities of antioxidative enzymes in patients with ischemic heart disease. Kardiologiia. 1992;32(6):25–27. 39. Seriakov VV, Sidorenko BA, Efuni SN. Interaction of hyperbaric oxygenation with nifedipine and propranolol at the level of autonomic regulation of the heart in patients with angina pectoris. Kardiologiia. 1992;32(1):50–53. 40. Seriakov VV, Efuni SN, Sidorenko BA. [Hyperbaric oxygenation and antianginal preparations: the effect of a single combined use on the functional indices of the state of the heart in patients with angina pectoris]. Anesteziol Reanimatol. 1992;(2):9–13. [Article in Russian]. 41. Al-Waili NS, Butler GJ, Beale J, et al. Influences of hyperbaric oxygen on blood pressure, heart rate and blood glucose levels in patients with diabetes and hypertension. Arch Med Res. 2006;37(8):991–997. 42. Moon RE. Nitroglycerine: relief from the heartache of decompression sickness? J Appl Physiol. 2006;101(6):1537–1538. 43. Seriakov VV, Feofanova ID. [Hyperbaric oxygenation and antiaggregants: effects on platelet function in patients with ischemic heart disease]. Anesteziol Reanimatol. 1997;(2):31–33. [Article in Russian]. 44. Yamasaki H, Matsubara S, Sasaki I, et al. Retinal Artery embolization during carotid angioplasty and carotid artery stenting: case report. Neurol Med Chir (Tokyo) . 2009;49(5):213–216. 45. Bachand RT Jr, Somani P. Digitalis intoxication: protection with hyperbaric oxygen. Life Sci. 1967;6(7):739–742. 46. Torbati D. Heparin effects during hyperbaric oxygenation in rats. Life Sci. 1985;36 (2):147–151. 47. Grover I, Conley L, Alzate G, et al. Hyperbaric oxygen therapy for hepatic artery thrombosis following liver transplantation: current concepts. Pediatr Transplant. 2006;10(2):234–239. 48. Sever C, Kulachi Y, Acar A, Duman H. Frostbite injury of hand caused by liquid helium: a case report. EPlasty. 2010;10: e35. 49. Banerjee C, Woller SC, Holm JR, Stevens SM, Lahey MJ. Atypical calciphylaxis in a patient receiving warfarin then resolving with cessation of warfarin and application of hyperbaric oxygen therapy. Clin Appl Thromb Hemost. 2010;16(3):345–350. 50. Stuhr LE, Maehle BO. The effect of Losartan, an angiotensin II antagonist, on cardiac function, mass and morphology in rats after repeated hyperbaric exposures. Scand J Clin Lab Invest. 1997;57(3):253–261. 51. Yilmaz MI, Korkmaz A, Kaya A, et al. Hyperbaric oxygen treatment augments the efficacy of losartan regime in an experimental nephrotic syndrome model. Nephron Exp Nephrol. 2006;104(1):e15–22. 52. Estrada EJ, Valacchi F, Nicora E et al. Combined treatment of intrapancreatic autologus bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant. 2008; 17(12):1295–1304. 53. Karadurmus N, Sahin M, Tasci C, et al. Potential benefits of hyperbaric oxygen therapy on atherosclerosis and glycaemic control in patients with diabetic foot. Endokrynol Pol. 2010;61(3):275–279. 54. Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;14(4):CD002967. 55. IMS Health. IMS Health Reports U.S. Prescription Sales Grew 5.1 Percent in 2009, to $300.3 Billion. Press release. Available at: https://www.imshealth.com/media. Accessed: April 10, 2010. 56. Wong T, Wang CJ, Hsu SL, et al. Cocktail therapy for hip necrosis in SARs patients. Chang Gung Med J. 2008;31(6):546–553. 57. Johnson GA, Gutti VR, Loyalka SK, et al. Albuterol metered dose inhaler performance under hyperbaric pressures. Undersea Hyperb Med. 2009;36(1):55–63. 58. Beckman DL, Crittenden DJ. Protection from oxygen-induced seizures by clonazepam and propylene glycol. Proc Soc Exp Biol Med. 1981;168(1):45–48. 59. Sonmez A, Yilmaz MI, Korkmaz A, et al. Hyperbaric oxygen treatment augments the efficacy of cilazapril and simvastatin regimens in an experimental nephritic syndrome model. Clin Exp Nephrol. 2008;12(2):110–118. 60. Sumen-Secgin G, Cimsit M, Ozek M, Eroglu L. Antidepressant-like effect of hyperbaric oxygen treatment in forced-swimming test in rats. Methods Find Exp Clin Pharmacol. 2005;27(7):471–474. 61. Alvernia JE, Patel RN, Cai DZ, et al. A successful combined endovascular and surgical treatment of a cranial base mucormycosis with an associated internal carotid artery pseudoaneurysms. Neurosurgery. 2009;65(4):733–740. 62. Dusoleil A, Eugène C, Wesenfelder L, Rocher P. Post-radiation duodenal ulceration treated with hyperbaric oxygen. Gastroenterol Clin Biol. 1994;18(2):172–174. 63. Kidd PM. Multiple sclerosis, an autoimmune inflammatory disease: prospects for its integrative management. Altern Med Rev. 2001;6(6):540–566. 64. Boschetti M, De Lucchi M, Giusti M, et al. Partial visual recovery from radiation-induced optic neuropathy after hyperbaric oxygen therapy in a patient with Cushing disease. Eur J Endocrinol. 2006;154(6):813–818. 65. Jian X, Guo G, Ruan Y, Lin D, Zhao B. Severe keloids caused by hydrogen cyanide injury: a case report. Cutan Ocul Toxicol. 2008;27(2):97–101. 66. Hart GB, Thompson RE. The treatment of cerebral ischemia with hyperbaric oxygen (OHP). Stroke. 1971;2(3):247–250. 67. Can hyperbaric oxygen improve erectile function following surgery for prostate cancer (HBOT)? Available at: https://clinicaltrials.gov/ct2/show/NCT00906269. Accessed: December 17, 2010. From the Shoe String Podiatry, Ormond Beach, Florida Address correspondence to: Robert G. Smith, DPM, MSc, RPh, C.Ped Shoe String Podiatry 723 Lucerne Cir. Ormond Beach, FL 32174 Email: Robert.Smith@fhmmc.org

















